|
|
|
   |
|
| ORIGINAL ARTICLE | |
|
Comparison of clinical and microbiological status of osseointegrated dental implant with natural tooth
Arun Kumar, Abhishek Kawadkar, Dimple Mathew, Shashikanth Hegde, Rajesh Keshyop Shankar
Department of Periodontology, Yenepoya Dental College, Mangalore, Karnataka, India
| Date of Web Publication | 15-Feb-2018 |
Correspondence Address:
Dr. Dimple Mathew
Department of Periodontology, Yenepoya Dental College, Deralakatte P.O, Mangalore – 575 018, Karnataka
India
Source of Support: None, Conflict of Interest: None
 |
Check |
DOI: 10.4103/jdi.jdi_3_17
 Abstract Abstract |
Aims: This study is aimed at investigation of microflora around healthy implants and comparison of the clinical and microbiological status of osseointegrated dental implant with that of a natural tooth.
Materials and Methods: Ten patients with healthy osseointegrated dental implants were further subdivided into two groups according to sites evaluated for clinical and microbiological parameters, Group A-Subgingival site corresponding to periimplant mucosa. Group B – subgingival site corresponding to natural tooth distal to implant. Oral prophylaxis was carried out for all the subjects at baseline, and they were recalled after 3 months for the assessment of clinical parameters such as probing depth, sulcus bleeding index, and plaque index. Subgingival plaque samples were taken on the same day and subjected to microbiological analysis by polymerase chain reaction. Statistical analysis was done using Mann–Whitney test and Fisher’s exact test.
Results: There existed a definite correlation between clinical parameters such as sulcular bleeding index, plaque index, and periodontal pockets and the presence of these microorganisms, i.e., Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythia, and Aggregatibacter actinomycetemcomitans between the two groups.
Conclusions: Although the ecological environment around dental implant may differ, the microbial findings of implants are similar to that of the natural tooth.
Keywords: Dental implants, osseointegration, polymerase chain reaction
| How to cite this article: Kumar A, Kawadkar A, Mathew D, Hegde S, Shankar RK. Comparison of clinical and microbiological status of osseointegrated dental implant with natural tooth. J Dent Implant 2017;7:46-53 |
| How to cite this URL: Kumar A, Kawadkar A, Mathew D, Hegde S, Shankar RK. Comparison of clinical and microbiological status of osseointegrated dental implant with natural tooth. J Dent Implant [serial online] 2017 [cited 2018 Mar 28];7:46-53. Available from: http://www.jdionline.org/text.asp?2017/7/2/46/225409 |
 Introduction Introduction |
 |
The accumulation and maturation of bacterial plaque biofilm at the gingival margin are widely recognized as the primary etiological factor in the development of chronic periodontitis. Biofilms are fascinating structures and may be found virtually anywhere. Biofilm consists of one or more communities of microorganisms, embedded in a glycocalyx, that are attached to a solid surface. The functions of biofilm depend on the ability of bacteria and microcolonies within the biofilm to communicate with one another (Quorum sensing).[1] The human oral cavity is inhabited by more than 500 species of bacteria at 108–109 bacteria per milligram of dental plaque. A distinct difference exists between the composition of supragingival and subgingival plaque. Supragingival plaque exhibits the accumulation of predominantly Gram-positive cocci whereas subgingival plaque is characterized by flora predominated by Gram-negative anaerobic bacilli such as Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Fusobacterium nucleatum  and have frequently been isolated from periodontal lesions and shown to be related to the onset and progression of periodontal disease.[2] The oral environment is unique because it is the only area of the body where biomaterials are routinely placed for long-term use in nonsterile environment and colonization of oral biomaterials by various microbes is inevitable. The use of dental implant is now widespread as they are root analogs that are surgically placed into the jaw bone and used to support crowns, bridges, and dentures. The excellent biocompatibility of implant material results mainly from its vital surface characteristics such as surface roughness and modifications, surface free energy, chemical composition of surface, and implant abutment fit.
and have frequently been isolated from periodontal lesions and shown to be related to the onset and progression of periodontal disease.[2] The oral environment is unique because it is the only area of the body where biomaterials are routinely placed for long-term use in nonsterile environment and colonization of oral biomaterials by various microbes is inevitable. The use of dental implant is now widespread as they are root analogs that are surgically placed into the jaw bone and used to support crowns, bridges, and dentures. The excellent biocompatibility of implant material results mainly from its vital surface characteristics such as surface roughness and modifications, surface free energy, chemical composition of surface, and implant abutment fit.
All these properties have created an impact on the biofilm formation on implant biomaterial.[3] Biofilm formation may cause inflammatory reactions around the implant (peri-implantitis) leading to implant failure. Hence, it is very important to develop implant surfaces (around transmucosal portion) that reduce the number of initially adhering bacteria, which minimizes biofilm formation and subsequent inflammation of the soft tissues. Implant material surface characteristically plays a vital role in effecting the biofilm formation and maturation.[3]
Periimplantitis is an inflammatory process affecting the tissues around an osseointegrated implant, resulting in the loss of supporting bone leading to implant failure. The factors associated with periimplantitis appear to be related to the composition of the bacterial environment around an implant and the ability of oral bacteria to adhere to the implanted biomaterial.[1] A certain micro roughness has been suggested to be appropriate for dental implants (Bollen, 1997) because surfaces below Ra 0.2 μm does not promote bacterial adherence due to the larger size of most microorganisms. Hence, it is important that commercially available dental implants should have a range of surface roughness/smoothness to reduce bacterial adhesion. Materials with surface energy of 20–30 dynes cm exhibit minimal biologic adhesiveness whereas higher surface energy support bioadhesion. Hence, smooth surface with low surface energy is demanded to minimize biofilm formation. Modifications are also made by chemically changing the surface to increase the antimicrobial capacity of titanium. Bacterial leakage influenced by implant-abutment fit results in an inflammatory cell infiltration in periimplant mucosa at borderline between abutment and implant.[4] Future of implant research will be in the development of scientific methods to evaluate both biomaterials and treatment designs based on desired biological outcomes. The physical and chemical composition is not end-point, but the beginning in the evaluation of biocompatible material. The understanding of pellicle formation as a function of surface composition, microbial adhesion to biomaterials, and cell reaction to implant biomaterials is not only necessary but essential.[4]
The most important goal of periodontal therapy is to reduce or eliminate the subgingival microorganisms associated with periodontal disease and to maintain the periodontal health. With the advent of implant dentistry, the current treatment for gingivitis and periodontitis which is directed at disruption of plaque biofilm maturation and/or reduction of the bacterial load may or may not hold true for implants unless we try and assess the biology and the pathology of periimplantitis.[3]
Currently, there is ample literature available on the microbiota of failing or failed implant but limited knowledge concerning the species of bacteria colonizing periimplant mucosa in successful osseointegrated implants. Hence, this study is aimed at investigation of microflora around healthy implants.
 Materials and Methods Materials and Methods |
 |
Patients for the study were selected from the Department of Periodontology, Yenepoya University, Mangalore; who have already undergone implant placement. Informed consent was obtained for participation in the study from all patients. Ethical clearance was obtained from Ethical Committee of Yenepoya University.
Selection criteria included (1) Partially edentulous patients aged between 20 and 50 years, (2) Patients with at least one dental implant, (3) No history of systemic diseases, (4) No history of systemic antibiotics/mouthrinse for last 3 months, (5) No history of smoking, (6) No history of periodontal therapy for last 3 months, and (7) No history of failed dental implants.
Ten patients selected for the study were considered for sampling under two groups Group A – subgingival plaque sample was collected from subgingival site around implant and Group B – subgingival plaque sample was collected from a subgingival site around natural tooth distal to implant. Following periodontal parameters were recorded: (1) Plaque Index (Silness and Loe).
(2) Sulcus bleeding Index (Mombelli et al. modification of Muhlemann and Son). (3) Probing pocket depth.
 Results Results |
 |
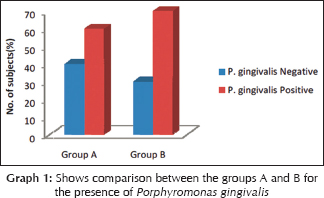
Correlation between clinical and microbiological findings for Porphyromonas gingivalis
The mean probing depth for P. gingivalis positive samples in Group A was 4.33 ± 0.816 and Group B was 3.78 ± 0.833. Z = 1.903 and P = 0.057 showing no statistically significant difference [Table 1]. The mean sulcular bleeding index score for P. gingivalis positive samples in Group A was 1.83 ± 0.753 and Group B was 1.00 ± 0.707. Z = 1.118 and P = 0.264 showing no statistically significant difference. The mean plaque index score for P. gingivalis positive samples in Group A was 2.13 ± 0.494 and Group B was 1.75 ± 0.530. Z = 1.396 and P = 0.163 showing no statistically significant difference [Graph 1].
 |
Table 1: Shows correlation between clinical and microbiological findings for P. gingivalis in both the groups |
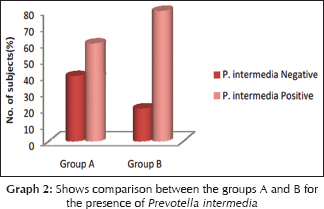
Correlation between clinical and microbiological findings for Prevotella intermedia
The mean probing depth for Prevotella intermedia positive samples in Group A was 4.17 ± 0.753 and Group B was 3.88 ± 0.835. Z = 0.688 and P = 0.491 showing no statistically significant difference [Table 2]. The mean sulcular bleeding index score for P. intermedia positive samples in Group A was 1.50 ± 1.049, and Group B was 1.00 ± 0.756. Z = 0.957 and P = 0.339 showing no statistically significant difference. The mean plaque index score for P. intermedia samples in Group A was 2.04 ± 0.459, and Group B was 1.72 ± 0.619. Z = 1.018 and P = 0.309 showing no statistically significant difference [Graph 2].
 |
Table 2: Shows correlation between clinical and microbiological findings for Prevotella intermedia |
Correlation between clinical and microbiological findings for Tannerella forsythia
The mean probing depth for Tannerella forsythia positive samples in Group A was 4.67 ± 0.577, and Group B was 4.50 ± 0.577. Z = 0.408 and P = 0.683 showing no statistically significant difference [Table 3]; the mean sulcular bleeding index score for T. forsythia positive samples in Group A was 2.00 ± 0.00 and Group B was 1.50 ± 0.577. Z = 1.342 and P = 0.180 showing no statistically significant difference. The mean plaque index score for T. forsythia positive samples in Group A was 2.33 ± 0.289, and Group B was 2.19 ± 0.239. Z = 0.764 and P = 0.445 showing no statistically significant difference [Graph 3].
 |
Table 3: Shows correlation between clinical and microbiological findings for T. forsythus |

Correlation between clinical and microbiological findings for Aggregatibacter actinomycetemcomitans
The mean probing depth for A. actinomycetemcomitans positive samples in Group A was 4.50 ± 0.707, and Group B was 4.50 ± 0.707. Z = 0.00 and P = 1.000 showing no statistically significant difference [Table 4].
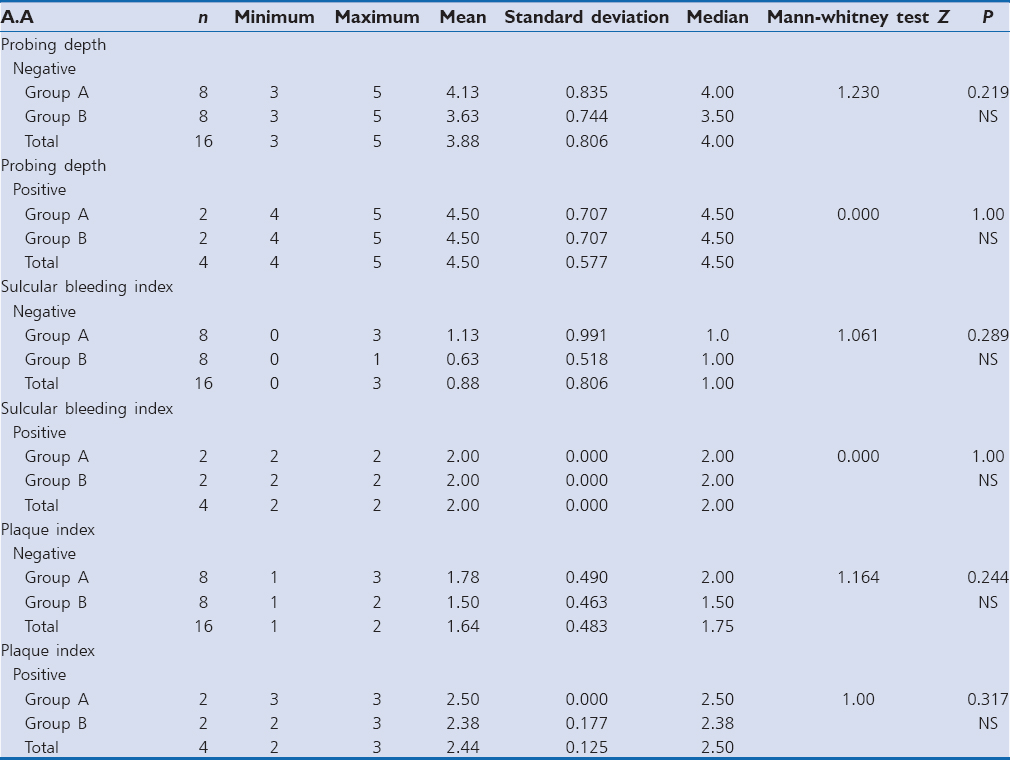 |
Table 4: Shows correlation between clinical and microbiological findings for Aggregatibacter actinomycetemcomitans |
The mean sulcular bleeding index score for A. actinomycetemcomitans positive samples in Group A was 2.00 ± 0.00 and Group B was 2.00 ± 0.00. Z = 0.00 and P = 1.00 showing no statistically significant difference The mean plaque index score for A. actinomycetemcomitans positive samples in Group A was 2.50 ± 0.00 and Group B was 2.38 ± 0.177. Z = 1.00 and P = 0.317 showing no statistically significant difference [Graph 4].

 Discussion Discussion |
 |
Dental implants are widely used mode of treatment of edentulism in which a titanium root analog is inserted into the bone followed by fabrication of superstructure once the implant osseointegrates.
Although it is clear that multiple factors can contribute to implant failure, an increasing number of studies point to the detrimental effect of anaerobic plaque bacteria on periimplant tissue health. There are essentially five lines of evidence supporting the view that microorganisms play a major role in causing periimplantitis:
- An experiment in humans, showing that deposition of plaque on implants can induce periimplant mucositis
- The demonstration of distinct quantitative and qualitative differences in the microflora associated with successful and failing implants
- Placement of plaque-retentive ligatures in animals leading to shifts in the composition of the microflora and periimplantitis
- Antimicrobial therapy improving the clinical status of periimplantitis patients
- Evidence that the level of oral hygiene has an impact on the long-term success of implant therapy.[5]
The microbial findings indicated that microbes of periimplant mucosa were similar to that of the gingival sulcus surrounding natural tooth. This finding is well supported by many studies done previously by Bauman et al.,[6] Mombelli,[7] Kohavi et al.,[8] Andrea George et al.,[9] and Heydenrijk et al.[10] All these studies concluded that the presence of microbial species depend on the ecological factor provided by artificial gingival crevice of permucosal implants in edentulous mouth before the placement of implants and is similar to that of the natural dentition in partially edentulous subjects.
The results also revealed the presence of P. gingivalis, P. intermedia in majority of subjects whereas T. forsythia and A. actinomycetemcomitans were present in only a few cases. These results are in agreement with the findings of Palmisano et al.,[11] Lee et al.,[12] and van Winkelhoff et al.,[13] who concluded that subgingival microbiota around periimplant mucosa consists of P. gingivalis, P. intermedia, and moderate amount of T. forsythia and A. actinomycetemcomitans.
This study also showed correlation between clinical parameters such as sulcular bleeding index, plaque index, and periodontal pocket depth and the presence of these microorganisms, i.e., P. gingivalis, P. intermedia, T. forsythia, and A. actinomycetemcomitans. This result is in accordance with the findings of Nakou et al.[14] and George et al.[9] The results of these studies clearly demonstrated that clinical and microbiological parameters are interrelated and deeper probing depths and gingival inflammation were found to be associated with red complex organisms.
We have come across the studies that tried to correlate the microbiological samples of implant and natural teeth. One such study by Nakou et al.[14] which assessed the microbial composition of implants before insertion and 2–10 weeks postinsertion showed that supra gingival plaque of implants was dominated by Gram-positive cocci and rods and subgingival by hemophilus sp and Veillonella parvula. They concluded that the presence of these species depends on the ecological factors provided by artificial gingival crevice of permucosal implants in edentulous mouth.
Along the similar lines, different techniques have provided us with different results in the quest for implant microbiota. Most review articles have reported that the microbiota of oral cavity before the implant placement determines the composition of microflora in periimplant area Heydenrijk et al.[10] which was well supported by Bauman,[6] who reviewed plaque-induced inflammation around dental implants and suggested that the microflora around successful implants is similar to healthy sulci while that associated with failing implants is similar to periodontally diseased sites, and implant microflora is similar to the tooth microflora in the partially edentulous mouth but widely differs from that of edentulous mouths This seems to indicate a bacterial reservoir around the teeth and the possibility of reinfection of the implant sulcus by periodontal pathogens and they concluded that periodontal and implant maintenance are linked and neither can be overlooked.
Kohavi et al.[8] using selective culturing methods found no significant difference between the subgingival flora around the teeth as compared to the implant flora. However, composition of the supragingival plaque was found to be different. Mombelli [7] stated that clinically stable implants showed no significant shifts in composition of flora over a period of 5 years whereas subjects with bone loss and pocket formation around implants: gram-negative anaerobic bacteria, particularly fusobacteria, spirochaetes, and black-pigmented species such as P. intermedia were found in higher proportions.
In the present study, endosseous implants were placed and assessed for microbiological analysis 3 months postoral prophylaxis for the presence of P. gingivalis, P. intermedia, A. actinomycetemcomitans, and T. forsythia in subgingival plaque samples taken from the periimplant tissue and were evaluated using polymerase chain reaction (PCR). Our findings were in accordance with these studies as our data also revealed that the correlation exists between microbes of natural teeth and implants.
There are different studies which have evaluated the periimplant plaque at different point time. Study by Koka et al.[15] revealed that initial colonization of marginal implant plaque occurs within 14 days whereas subgingival colonization takes around 28 days whereas according to Nakazato et al.,[16] it takes only 4 h for supragingival colonization to take place on implant surface in our study. Nakou et al.[14] assessed microbial composition of implants before insertion and 2–10 weeks postinsertion of implants. van Winkelhoff et al.[13] studied microbial colonization at baseline, 6, and 12 months. Nakazato et al.[16] studied in vivo plaque formation at 4 and 48 h whereas Quirynen et al.[17] studied at 3 month interval.
In our study, the microbial sampling was done only for subgingival microorganisms, and hence, the samples were collected at the interval of 3 months postoral prophylaxis.
Limitations of the study
Our study population comprised of only 10 individuals; hence, the results obtained may not show the general trends. Our evaluation period was only 3 months, and there was no follow-up. The microbiological analysis was qualitative and quantitative analysis was not done. In addition, subgingival plaque samples were sent for assessment using transport medium which took good 16–18 h before the samples were processed. Subgingival plaque samples were assessed only by PCR whereas different techniques were found superior for different organisms.
 Conclusions Conclusions |
 |
From the observations of this study, the following conclusions may be drawn:
- Microbial findings of periimplant mucosa were similar to that of the gingival sulcus surrounding natural tooth
- The same microbes suspected to be the main cause of periodontal destruction were found in the periimplant environment, i.e., P. gingivalis, P. intermedia, T. forsythia, and A. actinomycetemcomitans
- When periimplant microbiota was compared with the clinical parameters, the presence of P. gingivalis, P. intermedia, T. forsythia, and A. actinomycetemcomitans was generally consistent with the scores of probing depth, bleeding on probing and plaque index. This data suggests that these microbes have some role to play or can be suspected to be associated with
- Periimplant pathology.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
 References References |
 |
| 1. | |
| 2. | |
| 3. | |
| 4. | |
| 5. | |
| 6. | |
| 7. | |
| 8. | |
| 9. | |
| 10. | |
| 11. | |
| 12. | |
| 13. | |
| 14. | |
| 15. | |
| 16. | |
| 17. |
| Tables |
[Table 1], [Table 2], [Table 3], [Table 4]
|


